Gardener’s Mailbag: Neil Sperry - Tyler Morning Telegraph
Gardener’s Mailbag: Neil Sperry - Tyler Morning Telegraph |
- Gardener’s Mailbag: Neil Sperry - Tyler Morning Telegraph
- Highly reactive form of magnesium stabilized by bulky ligands - Nature.com
- Ramadan 2021: 4 easy-peasy, delicious recipes for Sehri and Iftar to try on Ramzan - Jagran English
| Gardener’s Mailbag: Neil Sperry - Tyler Morning Telegraph Posted: 27 Apr 2021 05:57 PM PDT Dear Neil: This happened to my Mexican plum last summer. What caused it, and what can I do to prevent it this year? Answer: At first glance, I thought this had been caused by caterpillars, but then I decided to Google "Texas A&M plant pathology bacterial leaf spot Mexican plum." Sure enough, as with domesticated varieties, this disease causes leaf spots that later turn brown and drop out. Their report does say that chemical control is not highly effective on fruit-producing varieties, so I can't get you much farther down the road, but at least you have an ID and a resource. Dear Neil: I have a vine that seems to crop up everywhere. (See photo of it in a stone path.) How can I eliminate it? Answer: The photo is blurry, but it appears to be some type of ivy (genus Hedera). I'd suggest that you try a broadleafed weedkiller (containing 2,4-D) applied directly and only to the ivy. Dear Neil: I had a large area of my lawn that I had covered with lantana. We were trying to cut down on the amount of grass we were maintaining. Now the lantana looks like it's not coming back, and the city is citing me. What should I tell the inspector? Answer: You're probably going to have to replace the lantana if it hasn't resprouted by now. Granted, shrubs and perennials were slow to leaf out because of continued cool weather this spring, but enough time has passed. Turf is usually the lowest maintenance living cover for most landscape areas, so maybe you could consider going back to a compromise of some turf and some lantana or other flowering perennials. Dear Neil: We have a large post oak in the Hill Country that we have lost to Hypoxylon canker. Can we use the chips from having the stump ground in our compost back at home? Is there any danger of spreading the disease in that way? Answer: I spent some time on university plant pathology websites trying to find a good answer for you. The Texas A&M Forest Service has a fact sheet on Hypoxylon canker that tells us that the spores are rather omnipresent in wooded areas and that it's almost impossible to keep trees from being exposed to them. It's when trees are weakened, for example, by drought, that the disease takes over. It sounds like the chips should be fine in your compost. You're welcome to do some more searching online, however. I only spent 30 minutes on it. That doesn't make me an expert. Dear Neil: We didn't get our cast iron plants protected like you did, so ours turned brown. Should they be cut back just above ground line? Answer: They should be cut back, but they have started to grow by now, so you can't cut them that low or you'll be cutting new leaves. Trim just above the new growth. Dear Neil: We have Turk's cap planted beneath live oaks. We'd like to put mulch down around them for the good looks it brings. However, there are a couple of inches of live oak leaves on the ground there now. Must they be removed first. I know they're serving the same purpose, but we both prefer the looks of the mulch. Answer: I would remove live oak leaves. They don't break down very rapidly. They will pack down and form an almost impenetrable layer of organic matter – like a thatch roof. Wait for a warm, dry day and just blow them out. It should be fairly easy. Dear Neil: My sago palm is growing new leaves. It survived the cold and snow. Don't give up on them! Answer: The sago and palm experts told us clear back in February that it might be May or even June (on the taller types) before we would know if they had survived. So glad yours has! I'll bet the layer of snow was just the insulation it needed. That's been the case for many types of low-growing plants. Dear Neil: I have a Brown Turkey fig that survived the cold and is growing well. However, it has no figs this year. Do you think they will sprout later, or will they come back next year? I've always gotten a good crop. Answer: It will be fine, but 2021 will probably not see you harvesting figs from it. You're just lucky it made it through the cold. Many people would consider that a major victory, as they're having to start over with new fig plants. Dear Neil: I've attached a photo of a three-shrub "hedge" of loropetalum in front of our house. It's done well for 12 years until last fall. It started to show signs of declining, and then February seemed to kill it. Now it's trying to come back with small buds. Should I wait on it? Answer: If you had told me only about the winter damage, I'd be tempted to give it that second chance, but since it seemed to be going downhill prior to winter, it may have run out of steam, either because the soil wasn't to its liking or because it had been pruned repeatedly to the same height and may have simply worn out. It's probably time to start over. If you were happy with it in the first place you could rework the bed and replant with more loropetalums, or you could change over to dwarf hollies, dwarf abelias, boxwoods or some other low-growing plant. Dear Neil: My Shumard red oak is only leafing out on one side. The other side has no leaves at all. Is that because of the February cold? Will it all catch up? Is there anything I need to do or anyone I need to call? Answer: To your first question, yes, that's due to the cold, and it's a phenomenon we're seeing all across Texas. Red oaks have been completely erratic. Many have leafed out entirely normally. Some still have absolutely no leaves. Many have leaves on portions of the tree like you're describing. The state's finest arborists and foresters tell me, however, that we all need to sit back and relax for a few weeks. We need to let Nature take its course – that most of this will even out and most of the trees will be back to normal by the time we get into summer. There is nothing we can or should do to speed it along or to guarantee their success. It just is what it is. Above all, however, do not have them pruned now. For one thing, this is the prime season for the spread of the oak wilt fungus, so you really don't want to expose their internal wood to that fungus by pruning before mid-July. Plus, most of the pruning will end up not being necessary in the first place. Have a question you'd like Neil to consider? Mail it to him in care of this newspaper or e-mail him at mailbag@sperrygardens.com. Neil regrets that he cannot reply to questions individually. |
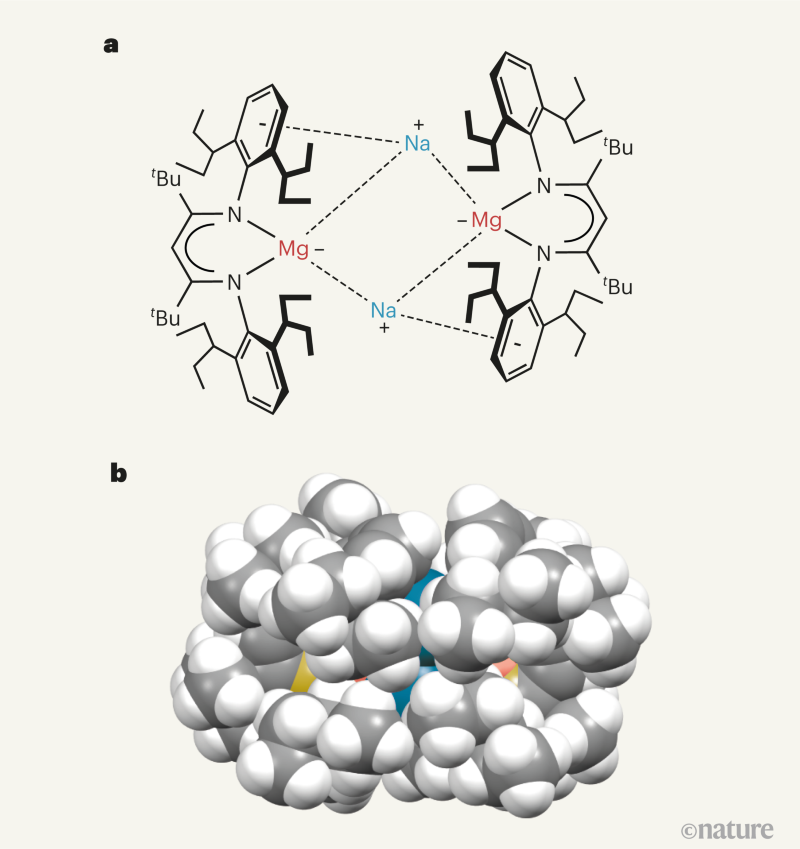
| Highly reactive form of magnesium stabilized by bulky ligands - Nature.com Posted: 28 Apr 2021 08:10 AM PDT NEWS AND VIEWS In the past few decades, innovative strategies have been developed to stabilize chemical compounds that contain main-group elements in unusually low oxidation states. (Main-group elements are those on the left and right sides of the periodic table, surrounding the transition metals.) Many of these compounds were previously thought to be impossible to obtain as stable materials. This is especially the case for magnesium, a reactive metal whose compounds almost all contain the element in the +2 oxidation state (equivalent to the atoms losing two electrons and having a charge of +2). Writing in Nature, Rösch et al.1 report that magnesium can form compounds in which it keeps all of its electrons, and thereby exists in the zero oxidation state that is normally characteristic of the pure element. These magnesium(0) compounds are stable at room temperature, but are highly reactive, with the metal atoms readily surrendering electrons in chemical reactions that are potentially useful for organic synthesis. It is well established that transition metals can exist in a range of oxidation states in their compounds, including the zero oxidation state. This characteristic is central to the ubiquitous application of such compounds as industrial catalysts. By contrast, the highly reactive s-block metals (groups 1 and 2 of the periodic table, excluding hydrogen) were not thought to be capable of forming similar, stable low-oxidation-state compounds, because they tend to lose all of their outermost electrons during compound formation. This idea was overturned in 2007, when the first compounds of magnesium — a group 2 metal — were prepared in the +1 oxidation state2. These compounds consist of two magnesium atoms connected by a chemical bond, with each magnesium atom bound to a bulky organic ligand; the ligand acts as a protective coating that prevents the magnesium ions from reverting to the +2 oxidation state. Other examples of compounds containing group-2 metals in low oxidation states have since been reported, such as beryllium in the zero3 and +1 oxidation states4, and calcium in the +1 state5, but they typically require special electron-accepting ligands to stabilize the compound concerned. Such advances in the chemistry of s-block metals have inspired chemists to pursue one of the outstanding goals in the field: the preparation of stable magnesium(0) compounds6. The logical approach would be to react an extremely bulky magnesium(ii) precursor compound with a powerful reducing agent, such as sodium or potassium metal. The idea is that the reducing agent would deliver two electrons to the magnesium atom, thus forming a magnesium(0) compound. In pursuit of this goal, researchers from the same laboratory as Rösch et al. previously developed an extraordinarily bulky ligand (abbreviated as BDI*), which they used to prepare a magnesium(ii) precursor compound7. However, when they reacted the precursor with potassium metal, only one electron was donated to the magnesium atoms, resulting in the formation of the magnesium(i) compound (BDI*)Mg–Mg(BDI*). Rösch and colleagues now report that, when they change the reducing reagent to a finely divided (powdered) form of sodium metal, two electrons are donated to the precursor, yielding the remarkable magnesium(0) compound {[(BDI*)Mg–][Na+]}2 (Fig. 1). It is clear that the enormous size of the BDI* ligand is required to stabilize the magnesium(0) compound, preventing the solid form of the compound from decomposing at room temperature. Even so, the compound partially decomposes at room temperature when in solution. This process yields another unprecedented type of compound known as a magnesium cluster, in which three magnesium atoms are bonded together, (BDI*)Mg–Mg–Mg(BDI*). Astonishingly, this contains magnesium in both the 0 and +1 oxidation states. Rösch et al. reasonably posit that the formation of this compound could shed light on the unknown mechanism of formation of Grignard reagents — an important class of magnesium(ii) compound that has been widely used in organic chemistry for more than 120 years. The size and 3D shape of the BDI* ligand hits the sweet spot when it comes to stabilizing the magnesium(0) compound. The authors report that the compound consists of a central core of magnesium and sodium atoms — [Mg2Na2]2+ — arranged in a ring and enveloped by two BDI* ligands (Fig. 1). Computational analyses reveal that the magnesium atoms have the same number of electrons as magnesium metal, which means that the compound could be viewed as a soluble form of the metal. There is, however, some sharing of electrons between the magnesium and sodium atoms. This doesn't detract from the assignment of the oxidation state of the magnesium atoms as zero, and the observation of a magnesium–sodium 'bond' in the compound is itself another first. Given that the magnesium atoms are in the zero oxidation state, the compound should display a level of reactivity similar to that of the elemental metal. In fact, Rösch and co-workers' preliminary experiments show that it is even more reactive than that. For example, it can readily activate (break or weaken) very strong bonds, such as hydrogen–hydrogen and carbon–fluorine bonds, at room temperature. Many other compounds that contain main-group elements in low oxidation states can do the same8. A true demonstration of the exceptional reducing ability of the magnesium(0) compound would be the activation of even more staunchly inert molecules, such as dinitrogen (N2). This seems achievable, given the recent demonstration that dinitrogen can be activated by a transiently formed calcium(i) compound9. A more surprising aspect of the reactivity of Rösch and colleagues' compound is that its magnesium(0) atoms can transfer electrons to its sodium atoms, reducing them back to sodium metal. This seems counter-intuitive, because the reverse process — the reduction of magnesium(ii) to magnesium(0) by sodium metal — was used to make the magnesium(0) compound in the first place. The authors' experimental evidence backs up the observation that the sodium atoms are reduced, but more work is required to examine the processes by which this operates. Rösch and co-workers' stable magnesium(0) compound is a landmark in the chemistry of the s-block elements. It will fundamentally change chemists' views about what can be synthesized using these elements. Moreover, it will help to advance our understanding of — and raise questions about — the unusual 'non-classical' bonding in low-oxidation-state main-group compounds. The development of highly reducing magnesium(0) compounds might also pave the way to their use in chemical reactions that, at present, cannot normally be carried out with s-block metals. The future is surely bright for magnesium now that it has hit zero. Nature 592, 687-688 (2021) References
|
| Ramadan 2021: 4 easy-peasy, delicious recipes for Sehri and Iftar to try on Ramzan - Jagran English Posted: 13 Apr 2021 12:00 AM PDT Ramadan 2021 is here and what better to try and experiment with interesting, delicious recipes for Sehri and Iftari. Therefore, satiate your taste buds by trying out these 4 yummy dishes for the holy month. Scroll down to know Updated: Tue, 13 Apr 2021 07:51 AM IST New Delhi| Jagran Lifestyle Desk: The holy month of Ramzan has started. It is celebrated on the completion of nine months of the Islamic calendar. Considered the most pious months for all Muslims, Ramadan begins with devotees observing fast for 30 days, called roza, depending upon the sighting of the crescent moon. As per Islamic beliefs, Quran came to earth from heaven and introduced itself to Prophet Muhammad. In 2021, Ramzan will start from April 12, that is Monday. According to Islam, keeping rozas helps to detoxify the soul and brings people closer to Allah. Only a two-time meal is allowed during this holy month, that is, Sehri (dawn) and Iftari (after sunset). It is said that adults and children who have to attain puberty and are mentally physically fit should observe this fast. Only the elderly and ill people are exempted from the fast. Not just this, even women who are pregnant, mensurating, and breastfeeding should not observe fast. They are all advised to observe the fast after they get recovered. Now, as the day is right around the corner, Muslims must be busy preparing for the same and must be mentally noting what can they cook for Sehri and Iftari that is not just tasty but also healthy. Well, it is very important to eat healthy food as the COVID-19 cases are witnessing a rapid surge. To ease down your task, we have brought you some simple recipes that will keep you healthy and energetic during your fasting hours. Khajur/Date Cake When it comes to commencing or breaking the fast, Khajur is an important fruit, as it is very nutritious, high in fibre, natural sweetener and many more. Prepare this easy recipe before Moon shines on Ramadan. Here have a look at the video: (video credit: Hebbars Kitchen) Salata/ Afghan Salad Salads are one of the healthy food items during Roza. Also, it's a very easy recipe that will help you save energy. Here have a look at the video: (video credit: Cook with Zahen) Banana Chia Smoothie Chia seeds are one of the healthy ingredients that you should add to your Sehri and Iftari. It'll not just keep you cool but is also rich in nutrients such as fibre, protein calcium, etc, and loaded with antioxidants. Added with the benefits of Banana, this shake is very healthy and will keep you going during the hours of Roza. It's easy to make, have a look at the video for the recipe: (video credit: Hamilton Beach India) Dry fruits Milkshake
(pic credit: Cooking with Siddhi) Rich with dry fruits, it is a healthy and easy recipe to have during Sehri and Iftari. To make this, you need to first soak all the dry fruits for at least 30 minutes, such as 7-8 dates, 1/4 cup almonds, 1/4 cup walnuts, 2-3 dry figs. After 30 minutes, drain all the water and put them in a blender, add 1 cup milk and 1 tbsp honey or jaggery. Blend it well until a thick paste now, add 2 cups of milk more and blend again. When done, pour it into a serving glass. Posted By: Niharika Sanjeeiv |
| You are subscribed to email updates from "whatcan i make with figs" - Google News.
| Email delivery powered by Google |
| Google, 1600 Amphitheatre Parkway, Mountain View, CA 94043, United States | |



Comments
Post a Comment